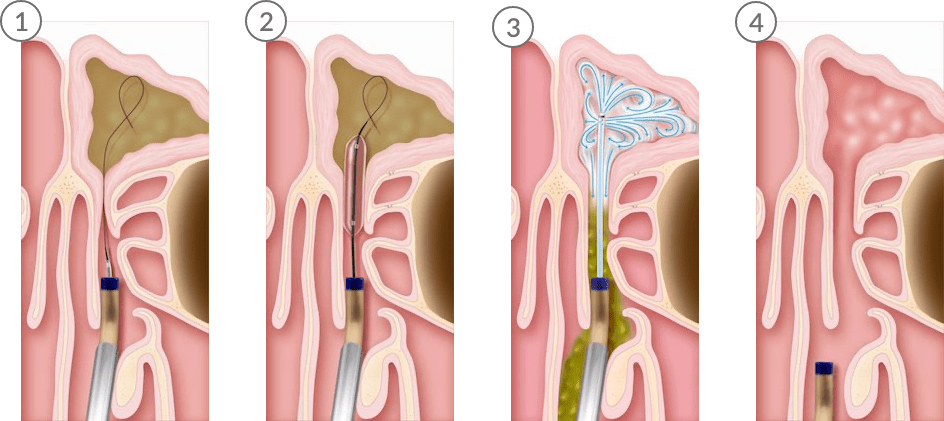
Balloon Sinuplasty opens blocked sinus cavities in four easy steps:
- A guide wire and balloon catheter are inserted into the inflamed sinus.
- The balloon is inflated to expand the sinus opening.
- Saline is sprayed into the infected sinus cavity to flush out pus and mucus.
- The system is removed, leaving the sinuses open.
What is Balloon Sinusplasty?

Balloon Sinuplasty is a breakthrough procedure that relieves the pain and pressure associated with chronic sinusitis. It is used by surgeons to safely and effectively treat chronic sinusitis patients who are not responding well to medications such as antibiotics, nasal steroids, or over-thecounter (OTC) drugs, and are seeking relief from uncomfortable and painful sinusitis symptoms.
Similar to how angioplasty uses balloons to open blocked arteries, Balloon Sinuplasty, a system of catheter-based instruments, opens blocked sinuses.
The procedure is less invasive than traditional sinus surgery. It allows most patients to return to normal activities quickly within 48 Hours.1
With Balloon Sinuplasty, a specially-designed catheter is inserted into the nose to reach the blocked sinus cavity. A small balloon is slowly inflated, which widens and restructures the walls of the sinus passage, helping to drain mucus from the blocked sinus and restore normal sinus drainage without cutting and with minimal bleeding. This approach also preserves the natural structure of the sinuses.
Published clinical data shows that Balloon Sinuplasty provides symptom relief for the majority of patients:
In the operating room:
- A multi-center study of 1,036 patients who had Balloon Sinuplasty reported that sinus symptoms improved in 95 percent of patients at an average follow-up period of 9 months.2
- Another multi-center study followed 65 patients who had Balloon Sinuplasty and reported clinically and statistically significant improvement in patient symptoms out to two years.3
In the office:

Acclarent office-based sinus dilation is:
- Safe and effective, with clinically and statistically significant improvement in symptom and radiographic scores • Practical, with 93.2% of sinuses successfully dilated and evidence suggesting suitability for patients with mild/ moderate ethmoid disease
- Compelling for patients, with the majority of patients returning to normal activity within 2 days of the procedure.4
When compared to Medical Management:
- In a multi-center study of 198 patients, who previously failed medical management, over 74 percent chose Balloon Sinuplasty over medical management alone.
- Balloon Sinuplasty provided superior Quality of Life outcomes over medical management across all three validated scales (SNOT20, CSS, RSDI).
- Balloon Sinuplasty as a stand-alone procedure also provided superior outcomes.
- There was a 97.6 percent technical success rate dilating the targeted sinuses.5
Balloon Sinuplasty is performed under general anesthesia in an outpatient setting; however, some surgeons are choosing to treat certain patients in their office under local anesthesia. The reported complication rate for Balloon Sinuplasty is low. Patients who suffer from chronic sinusitis and are not responding well to medications may benefit from seeing an Ear, Nose, and Throat (ENT) physician who performs Balloon Sinuplasty to determine if the procedure is right for them. Most insurance companies and Medicare provide coverage for Balloon Sinuplasty. Balloon Sinuplasty has been used to treat more than 510,000 patients, in over 560,000 procedures (over 88,000 procedures in the office) since receiving FDA clearance.6
Caution: Federal (US) law restricts the sale, distribution or use of these devices to, by or on the order of a physician. Third party trademarks used herein are trademarks of their respective owners. This site is intended for visitors from the United States and published by Acclarent, Inc., which is solely responsible for its contents.
Balloon Sinuplasty Technology is intended for use by or under the direction of a physician. Balloon Sinuplasty Technology has associated risks, including tissue and mucosal trauma, infection, or possible optic injury. Prior to use, it is important to read the Instructions for Use and to understand the contraindications, warnings and precautions associated with these devices.
Sources
1. Karanfilov B, Silvers S, Pasha R, Sikand A, Shikani A, Sillers M and the ORIOS2 Study Investigators. Office-based balloon sinus dilation: a prospective, multicenter study of 203 patients. Int Forum Allergy Rhinol, 2012; 00:1-8.
2. Levine et al, “Multicenter Registry of Balloon Catheter Sinusotomy Outcomes for 1,036 Patients.”Annals of Otology, Rhinology & Laryngology, 117(4):263-270, 2008.
3. Weiss et al. “Long-term outcome analysis of balloon catheter sinusotomy: Two-year follow-up.” Otolaryngology-Head and Neck Surgery (2008) 139, S38-S46.
4. Karanfilov B, Silvers S, Pasha R, Sikand A, Shikani A, Sillers M and the ORIOS2 Study Investigators. Office-based balloon sinus dilation: a prospective, multicenter study of 203 patients. Int Forum Allergy Rhinol, 2012; 00:1-8.
5. Payne, S. et al Medical therapy versus sinus surgery by using balloon sinus dilation technology: A prospective multicenter study, Am J Rhinol Allergy, Jun 17 2016, 1-8.
6. Acclarent Procedural Data Documented on 9-1-16. Acclarent, Inc. 33 Technology Drive Irvine, CA 92618 U.S.A. www.acclarent.com toll free +1-877-SPLASTY +1(877-775-2789) phone +1-650-687-5888 fax +1-650-687-5886
